
This is a book that students will not only value during their formal education, but will keep and refer to throughout their careers as chemists. Students are invited to enter the exciting world of chemical phenomena with a sound knowledge and understanding of the subject, to approach experimentation with an open mind, and to assess observations reliably. The chemistry of the elements is still discussed within the context of an underlying theoretical framework, giving cohesion and structure to the text, but at all times the chemical facts are emphasized. The authors have broken with recent tradition in the teaching of their subject and adopted a new and highly successful approach based on descriptive chemistry. Accordingly, the book covers not only the 'inorganic' chemistry of the elements, but also analytical, theoretical, industrial, organometallic, bio-inorganic and other cognate areas of chemistry. The authors specifically avoid the term `inorganic chemistry' since this evokes an outmoded view of chemistry which is no longer appropriate in the final decade of the 20th century.
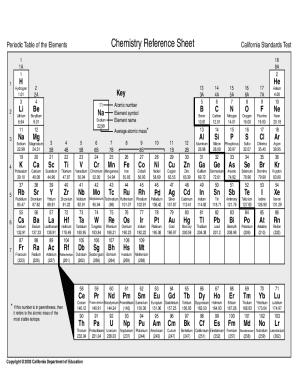
PERIODIC TABLE OF ELEMENTS CHEMISTRY REFERENCE TABLE FULL
This crucial central area of chemistry is full of ingenious experiments, intriguing compounds and exciting new discoveries. The book presents a balanced, coherent and comprehensive account of the chemistry of the elements for both undergraduate and postgraduate students. No page has been left unaltered but the novel features which proved so attractive have been retained. Now the authors have completely revised and updated the text, including more than 2000 new literature references to work published since the first edition. Earnshaw When this innovative textbook first appeared in 1984 it rapidly became a great success throughout the world and has already been translated into several European and Asian languages. The book closes with an examination of further chemical aspects including lesser known trends within the periodic system such as the knight's move relationship and secondary periodicity, as well at attempts to explain such trends.Ĭhemistry of the Elements by N. Finally, chapter 10 considers the way that the elements evolved following the Big Bang and in the interior of stars.
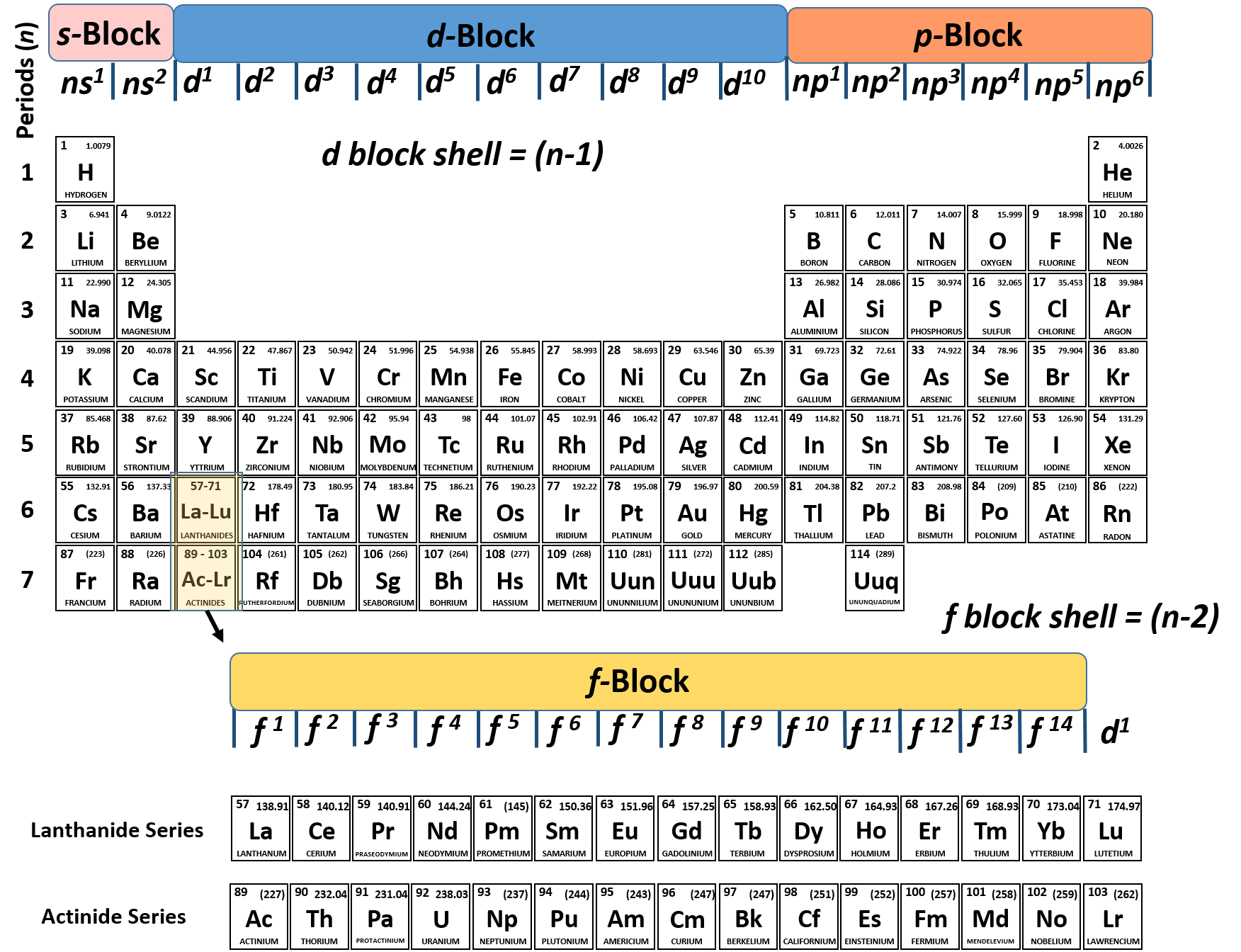
Chapter 9 provides a critical analysis of the extent to which modern quantum mechanics is, or is not, able to explain the periodic system from first principles. Chapter 8 discusses the response to the new physical theories by chemists such as Lewis and Bury who were able to draw on detailed chemical knowledge to correct some of the early electronic configurations published by Bohr and others. Chapters 6 and 7 consider the impact of physics including the discoveries of radioactivity and isotopy and successive theories of the electron including Bohr's quantum theoretical approach. Two chapters are devoted to the discoveries of Mendeleev, the leading discoverer, including his predictions of new elements and his accommodation of already existing elements. In chapter 3 the discovery of the periodic system by six independent scientists is examined in detail. The precursors to the periodic system, like Döbereiner and Gmelin, are discussed. The book then turns to a systematic account of the early developments that led to the classification of the elements including the work of Lavoisier, Boyle and Dalton and Cannizzaro. The Periodic Table begins with an overview of the importance of the periodic table and of the elements and it examines the manner in which the term 'element' has been interpreted by chemists and philosophers. The book is written in a lively style to appeal to experts and interested lay-persons alike. The present book provides a successor to van Spronsen, but goes further in giving an evaluation of the extent to which modern physics has, or has not, explained the periodic system. The one definitive text on the development of the periodic table by van Spronsen (1969), has been out of print for a considerable time. It lies at the core of chemistry and embodies the most fundamental principles of the field. Scerri The periodic table is one of the most potent icons in science.


 0 kommentar(er)
0 kommentar(er)
